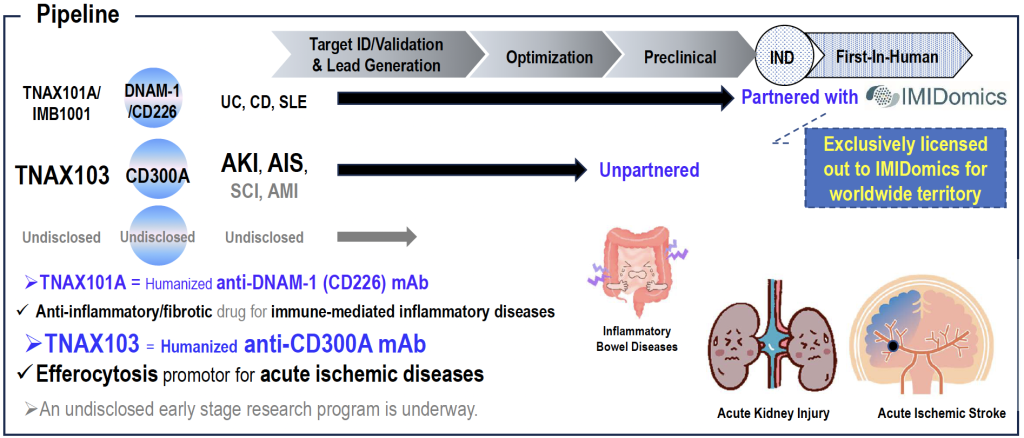
TNAX103, a humanized anti-CD300A mAb, is an efferocytosis promotor and the only anti-inflammatory neuroprotectant that fundamentally inhibits ischemia-reperfusion injury in acute ischemic stroke.
Problems of Current Treatment of AIS
Acute ischemic stroke (AIS) is a medical emergency caused by decreased blood flow to the brain, which results in damage to brain cells within minutes. Each year, AIS affects millions of people worldwide, with a significant impact on mortality and disability rates. Prompt treatment can reduce the brain damage and other complications, and the standard treatment for AIS is intravenous thrombolysis and endovascular thrombectomy.
These recanalization therapies, however, have issues. Abrupt reperfusion, in particular, often causes secondary brain damage. Ischemia-reperfusion injury (IRI) is caused by the rapid restoration of the blood supply to tissues after ischemia, but currently no effective treatment is available. A new treatment is needed to prevent IRI after AIS.
Inflammation in Dead Cells
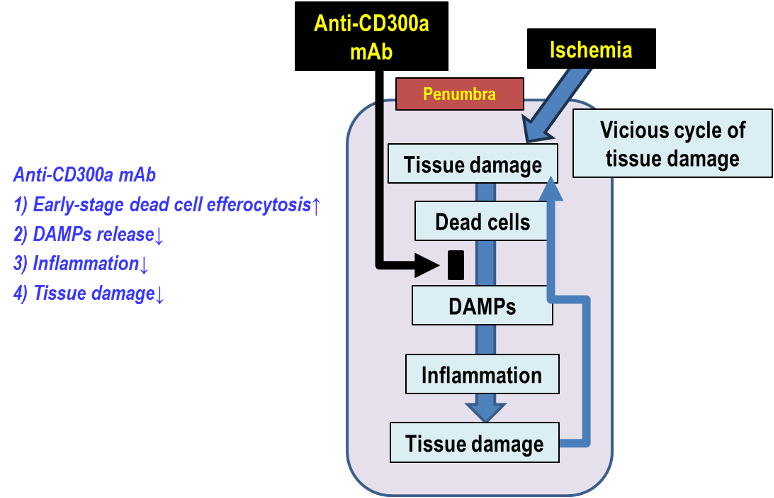
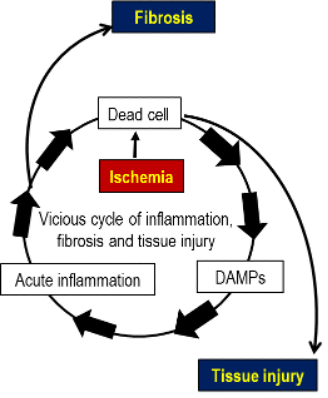
Inflammatory pathways begin hours or later after AIS and lead to neuronal cell death. Ischemic cell death induces inflammation, which results in further cell death and tissue damage in the penumbra (i.e., a vicious cycle of tissue damage). The penumbra, the reversibly damaged and perfused brain region surrounding the ischemic core, is a critical tissue at risk of cell death and can be rescued by controlling cell death-induced inflammation. Therefore, penumbra represents a new pharmacological target for AIS.
The vicious cycle of tissue damage in the penumbra:
“Tissue damage” -> ”DAMPs release from dead cells” -> “Inflammation” -> “Further tissue damage” [DAMPs (Damage-Associated Molecular Patterns) are released from ischemia-induced dead cells and produce proinflammatory cytokines.]
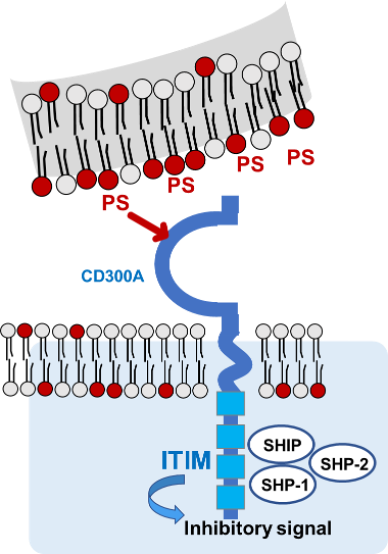
CD300A and PS
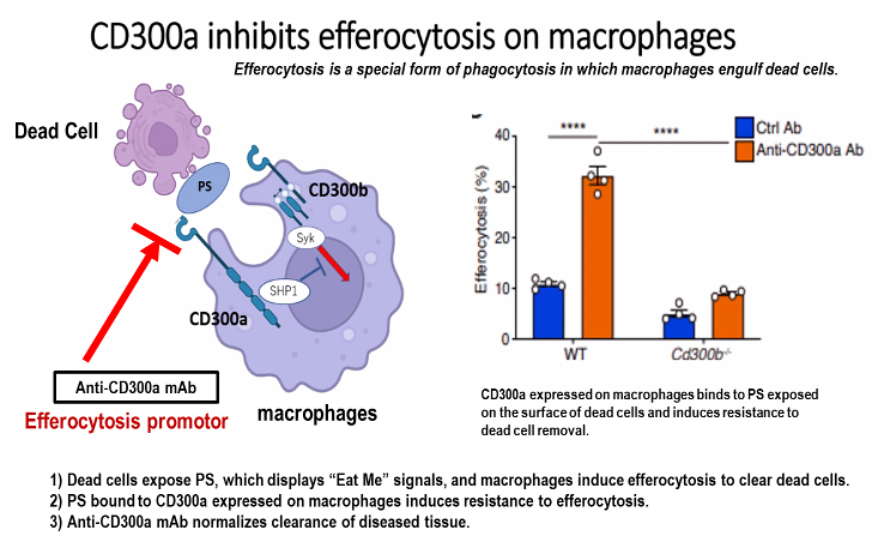
Normally, phosphatidylserine (PS) is confined to the inner membrane leaflet of viable cells, whereas in dead cells PS is exposed on the outer membrane. PS exposed on the outer membrane of dead cells acts as an “Eat Me” signal, and macrophages induce efferocytosis to remove the dead cells. On the other hand, CD300A expressed on macrophages is a key anti-efferocytic molecule. PS is a ligand for CD300A expressed on macrophages, and PS bound to CD300A expressed on macrophages induces resistance to dead cell removal (efferocytosis). Anti-CD300A (efferocytosis promotor) mAb blocks CD300A binding to PS and normalizes the clearance of the diseased tissues.
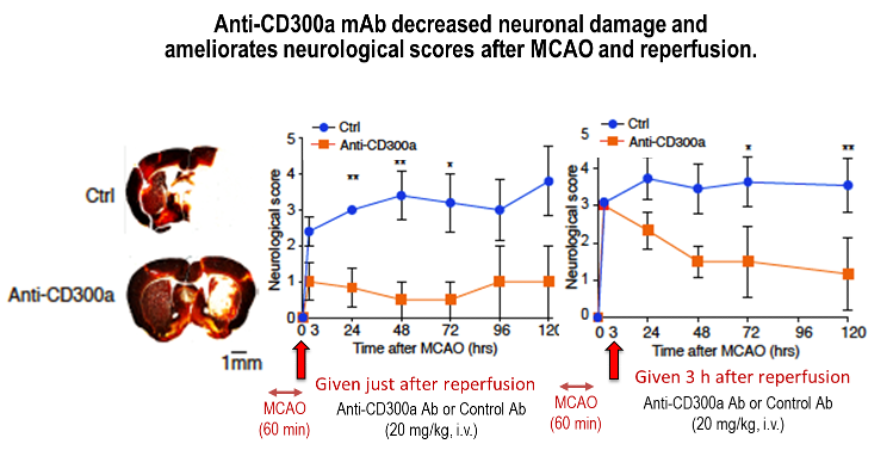
Pharmacological Effects of Anti-CD300a mAb in Mouse MCAO Model
Our anti-CD300a mAb decreases infarct volume and ameliorates neurological scores in mouse middle cerebral artery occlusion and reperfusion model.
TNAX103 is also believed to restore kidneys from AKI.
Acute Kidney Injury and Efferocytosis
Acute kidney injury (AKI) occurs in up to 7% of hospitalized patients and up to 30% of ICU patients, and mortality is very high (40-50% in hospitalized patients and >50% in ICU patients). AKI is a high risk factor for progression of CKD over time. The most common cause of AKI in hospitalized patients is acute tubular necrosis (ATN), and injury in the renal proximal tubular epithelium is often seen. Cell death of vascular endothelium and renal tubular epithelium plays an important role in the pathological progression from AKI to AKD to CKD. Inflammation after AKI causes renal dysfunction and fibrosis. Currently, there are no effective means of preventing or treating AKI.
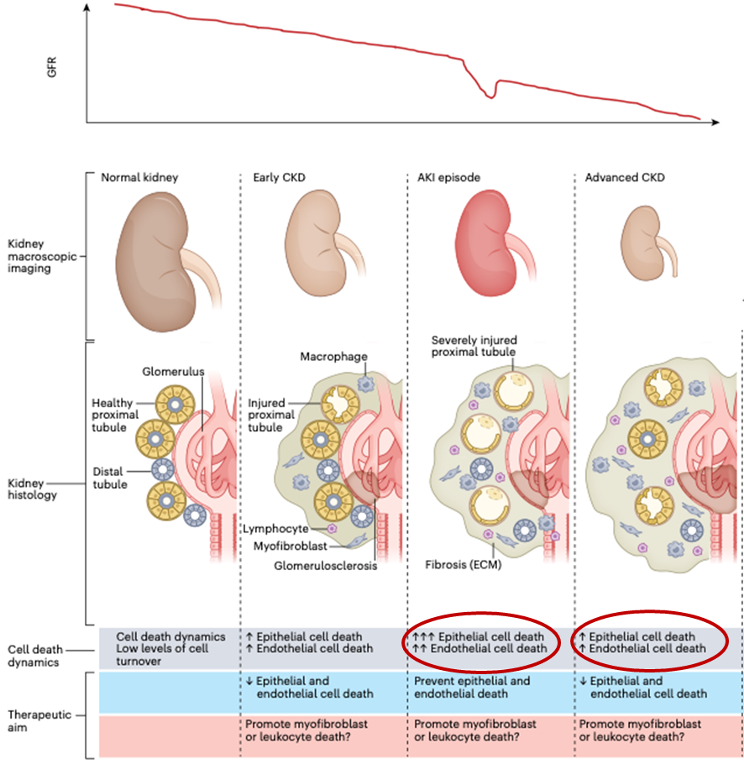
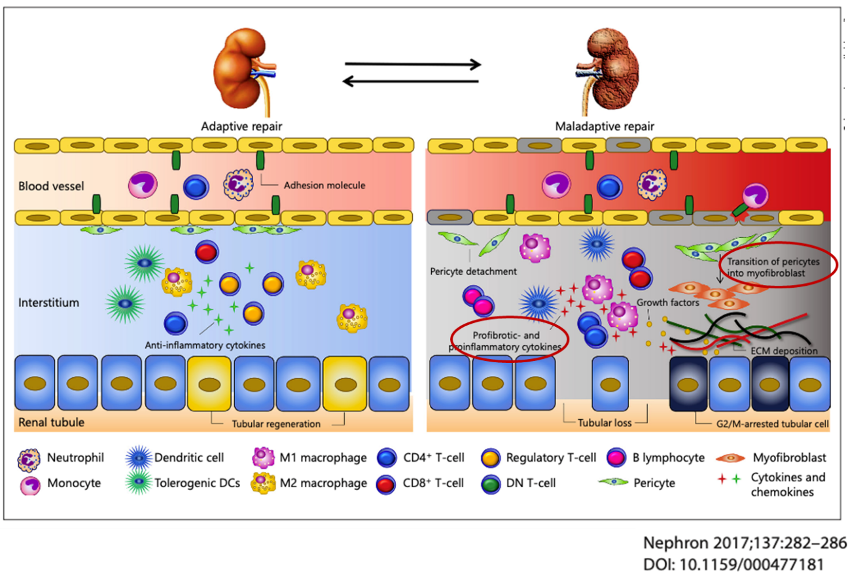
Efferocytosis promotors are expected to restore normal kidneys from AKI and AKD and prevent progression to CKD. Increased phagocytosis of dead cells by macrophages reduced DAMPs release, suppresses acute inflammation and prevents the vicious cycle of induction of further dead cells and tissue injury.
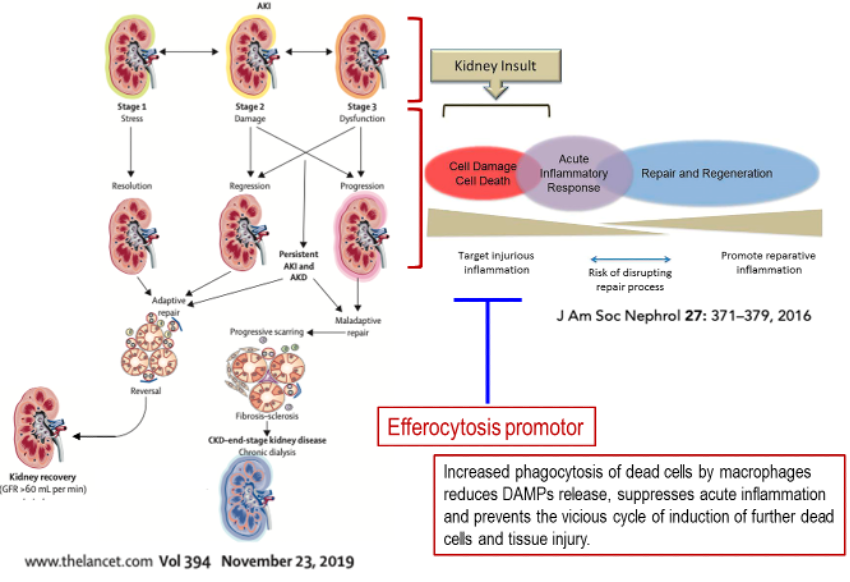
Effects of Anti-CD300a mAb in Mouse AKI Models
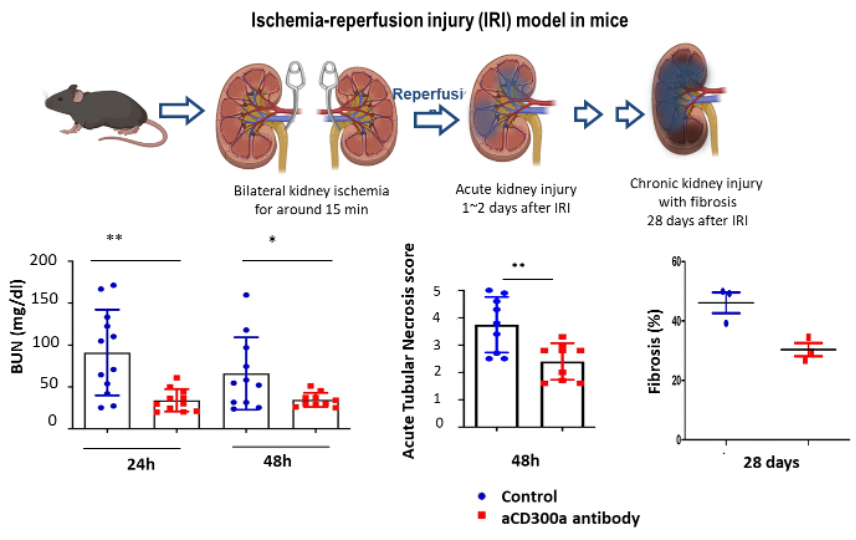
Our anti-CD300a mAb ameliorates renal function, tubular damage and fibrosis after ischemia-reperfusion injury (IRI) in mice.
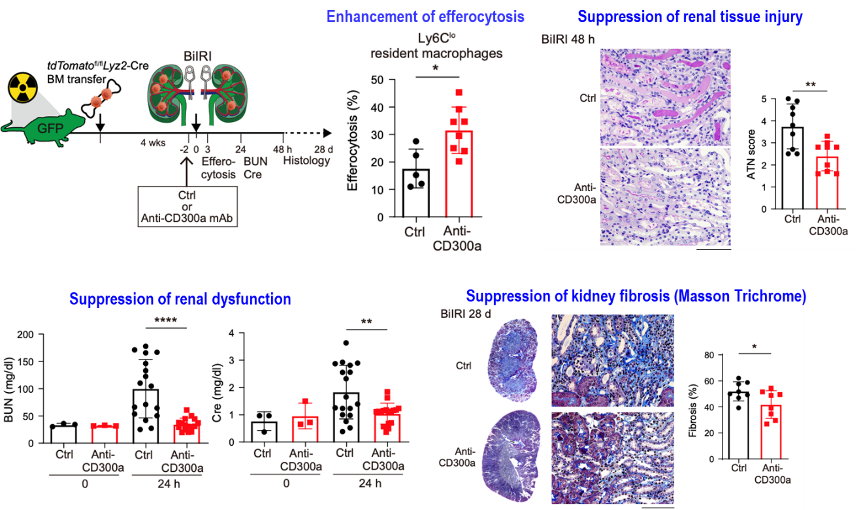
Our anti-CD300a mAb promotes efferocytosis and attenuates AKI and kidney fibrosis in another mouse model.
TNAX103 is also expected to be an efficacious treatment option for SCI and AMI.
There is no effective treatment for Spinal Cord Injury (SCI). In a mouse SCI model, our anti-CD300a mAb ameliorates locomotor ability and decreases areas of SCI and demyelination.
Our anti-CD300a mAb ameliorates acute myocardial infarction (AMI) induced by ischemia-reperfusion in mice.
TNAX101A (IBM1001), a humanized anti-DNAM-1 (CD226) mAb, is anti-inflammatory/fibrotic drug for the treatment of immune-mediated inflammatory diseases.
DNAM-1 (CD226) is a promoter of immune responses and produces inflammatory cytokines from immune cells including effector T cells.
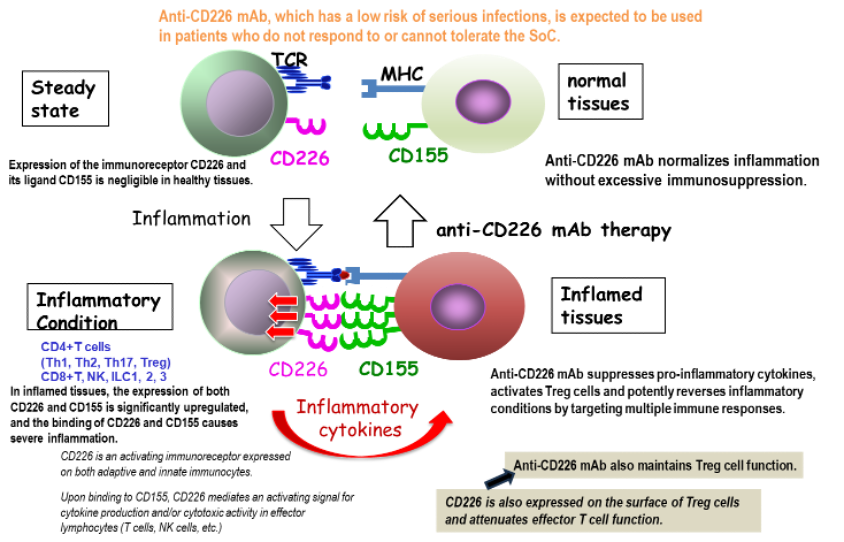
The clinical candidate is TNAX101A, renamed IMB1001. IMB1001 is considered to be a preferred treatment option for Crohn’s disease and SLE. Unlike other anti-inflammatory drugs, IMB1001 is not excessively immunosuppressive and carries a low risk of serious infection.
TNAX101A was discovered by TNAX Biopharma, and IMB1001 is currently being developed by our partner, IMIDomics.